中国防痨杂志 ›› 2025, Vol. 47 ›› Issue (9): 1105-1125.doi: 10.19982/j.issn.1000-6621.20250276
中国防痨协会多学科诊疗分会, 深圳市第三人民医院(国家感染性疾病临床医学研究中心), 首都医科大学附属北京朝阳医院, 广东省肺癌研究所
收稿日期:2025-07-01
出版日期:2025-09-10
发布日期:2025-08-27
基金资助:Multidisciplinary Diagnosis and Treatment Branch of Chinese Antituberculosis Association , National Clinical Research Center for Infectious Disease/Shenzhen Third People’s Hospital , Beijing Chao-Yang Hospital, Capital Medical University , Guangdong Lung Cancer Institute
Received:2025-07-01
Online:2025-09-10
Published:2025-08-27
Supported by:摘要:
肺结核与肺癌共病是指同一患者同时或先后罹患肺结核与肺癌两种疾病的状态。肺结核与肺癌共病的发生增加了诊断与鉴别诊断难度,易造成漏诊、误诊及治疗延迟。肺癌化疗、靶向药物与抗结核药物间存在复杂的相互作用,不仅影响抗结核和抗肿瘤的疗效,还显著增加药物的不良反应,亟需通过多学科协作,制定规范化诊疗共识,指导临床实践。为此,中国防痨协会多学科诊疗分会与国家感染性疾病临床医学研究中心(深圳市第三人民医院)共同牵头,联合首都医科大学附属北京朝阳医院、广东省肺癌研究所等,制定了《肺结核与肺癌共病诊疗专家共识》。本共识对肺结核与肺癌共病的流行病学特征、临床表现、诊断与治疗,以及结核分枝杆菌潜伏感染筛查与管理进行了阐述,重点围绕药物相互作用、治疗方案调整、手术与放疗时机把握等临床难点问题提出了解决方案,共提出了22条推荐意见,以期为临床实践提供规范化指引。
中图分类号:
中国防痨协会多学科诊疗分会, 深圳市第三人民医院(国家感染性疾病临床医学研究中心), 首都医科大学附属北京朝阳医院, 广东省肺癌研究所. 肺结核与肺癌共病诊疗专家共识[J]. 中国防痨杂志, 2025, 47(9): 1105-1125. doi: 10.19982/j.issn.1000-6621.20250276
Multidisciplinary Diagnosis and Treatment Branch of Chinese Antituberculosis Association , National Clinical Research Center for Infectious Disease/Shenzhen Third People’s Hospital , Beijing Chao-Yang Hospital, Capital Medical University , Guangdong Lung Cancer Institute . Expert consensus on the diagnosis and treatment of coexistent pulmonary tuberculosis and lung cancer[J]. Chinese Journal of Antituberculosis, 2025, 47(9): 1105-1125. doi: 10.19982/j.issn.1000-6621.20250276
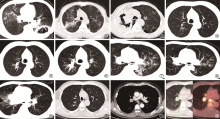
图1~12
肺结核与肺癌共病的影像学表现。图1 患者,男性,72岁,活动性肺结核,初治涂阳;CT检查显示左下肺巨大空洞,内壁不规则,洞内有分隔和粗大索状影;病理诊断鳞癌。图2、3 为同一患者,男性,58岁,涂阳肺结核;图2为患者抗结核治疗6个月,CT复查显示右上肺残留不规则实变,伴多发纤维条索影、结节影和支气管扩张影,符合结核病治疗后改变;图3患者为5年后CT检查,可见右上肺被巨大肿块所替代,内见不规则空洞形成;病理提示为鳞癌。图4~6为同一患者,既往肺结核病史;图4可见患者右肺上叶后端残留支气管扩张;图5显示患者7个月后支气管扩张区呈黏液嵌塞征表现,周围散在结节影;图6显示患者13个月后支气管扩张的管腔内软组织病变生长呈典型支气管铸型征表现;行支气管镜组织活检,病理诊断为鳞癌;同时,气道分泌物抗酸杆菌涂片阳性,提示结核病复发。图7~9为同一患者,诊断为左上叶活动性肺结核,涂阳,初治。图7为患者治疗前,右肺中间段支气管未见明显异常;图8为患者经抗结核治疗后病变逐渐吸收,但右肺中间段支气管略见狭窄,未采取措施;图9为患者抗结核治疗5个月后复查CT,可见肺结核病变显著吸收,中间段支气管管壁狭窄直至闭塞,不符合结核病转归病程,呈“矛盾现象”;支气管镜活检病理诊断为鳞癌。图10~12为同一患者。图10为患者右上叶肺腺癌(Ⅰb期)切除后肺部CT表现;图11为患者术后3个月复查CT,可见右肺门及隆突下淋巴结肿大;图12为患者PET/CT图像,可见纵隔及肺门淋巴结肿大,最大标准摄取值呈高摄取;经超声支气管镜淋巴结活检,提示为肉芽肿炎;组织标本送GeneXpert MTB/RIF检查提示结核分枝杆菌DNA阳性

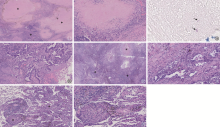
图13~20
肺结核与肺癌共病的病理鉴别。图13~19为同一患者,诊断为肺癌与肺结核共病;图13显示低倍镜下可见肉芽肿(*)和肿瘤(+)两种病变(HE染色 ×20);图14为肉芽肿,中央为干酪样坏死,周边多量淋巴细胞浸润(HE染色 ×100);图15为干酪样坏死物内找到抗酸染色阳性致病菌,确诊为肺结核(抗酸染色 ×1000);图16为肿瘤呈腺泡结构,细胞单层或复层排列,异型性明显,确诊为腺癌(HE染色 ×100)。图17、18为同一患者,肺结核伴肺泡上皮细支气管化生;图17显示低倍镜下见干酪样坏死性肉芽肿(*),经结核分枝杆菌检测确诊为肺结核,肉芽肿周围形态不规则的腺管状结构(+),疑似肺癌(HE染色 ×40);图18为腺管状结构,表面被覆Ⅱ型肺泡上皮细胞、Club细胞和纤毛细胞,确诊为塌陷的肺泡伴细支气管化生(HE染色 ×100)。图19、20为同一患者,肺癌伴肿瘤结节病样反应;图19显示低倍镜下见肉芽肿(*)和肺腺癌(+)(HE染色 ×100);图20为肉芽肿,圆形,边界清楚,中央无坏死,确诊为肿瘤结节病样反应(HE染色 ×200)

表1
肺癌化疗相关药物与利福平相互作用及使用建议
| 药品 | 药物代谢酶 | 与利福平相互作用 | 建议 |
|---|---|---|---|
| 紫杉醇类(含紫杉醇、紫杉醇脂质体、白蛋白紫杉醇、紫杉醇胶束) | CYP3A4/2C8 | 利福平诱导CYP3A4,加速紫杉醇类药物代谢,降低血药浓度,药品说明书建议谨慎合用 | A |
| 多西他赛 | CYP3A4/2C8 | 利福平诱导CYP3A4,导致多西他赛清除率增加,血药浓度下降。药品说明书建议谨慎合用 | A |
| 顺铂/卡铂 | 非CYP代谢 | 铂类药物通过DNA交联发挥作用,不依赖CYP酶代谢。药品说明书建议使用利福平无需调整剂量 | C |
| 培美曲塞 | 非CYP代谢 | 培美曲塞主要经肾排泄,利福平不影响其清除率。药品说明书建议无需调整剂量 | C |
| 吉西他滨 | 非CYP代谢 | 吉西他滨通过胞嘧啶脱氨酶代谢,利福平不影响该酶活性。药品说明书建议无需调整剂量 | C |
| 依托泊苷 | CYP3A4 | 利福平可能诱导CYP3A4,加速依托泊苷代谢,增加骨髓抑制风险。药品说明书建议谨慎合用 | A |
| 伊立替康 | CYP3A4 | 利福平诱导CYP3A4,增加伊立替康活性代谢产物SN-38生成,可能加重腹泻毒性。药品说明书建议谨慎合用 | A |
| 长春瑞滨 | CYP3A4 | 利福平诱导CYP3A4,可能降低长春瑞滨血药浓度。药品说明书建议谨慎合用 | A |
| 替莫唑胺 | 非酶水解 | 替莫唑胺在酸性条件下水解为活性产物,利福平不影响其分解过程。药品说明书建议无需调整剂量 | C |
| 芦比替定 | CYP3A4 | 芦比替定主要经CYP3A4代谢,利福平诱导酶活性可能降低其疗效。药品说明书建议尽量避免使用 | A |
| 芦康沙妥珠单抗 | TROP2抗体部分不依赖CYP代谢,细胞毒性部分(KL610023,拓扑异构酶Ⅰ抑制剂)由CYP3A4代谢 | 尚缺乏患者体内相互作用研究,但理论上利福平可能会削弱芦康沙妥珠单抗的疗效,建议谨慎合用 | B |
| 德曲妥珠单抗 | HER2抗体部分不依赖CYP代谢,细胞毒性部分(DXd,拓扑异构酶Ⅰ抑制剂)主要由CYP3A4代谢 | 尚缺乏患者体内相互作用研究,但理论上利福平可能会削弱德曲妥珠单抗的疗效,建议谨慎合用 | B |
表2
肺癌常用靶向治疗药物与利福平相互作用及使用建议
| 药品 | 作用靶点 | 药物代谢酶 | 与利福平相互作用及底物AUC变化 | 建议 | ||
|---|---|---|---|---|---|---|
| 奥希替尼 | EGFR | CYP3A4 | 说明书提示利福平诱导CYP3A4会显著降低其血药浓度。使奥希替尼的稳态AUC下降78%[ | A | ||
| 阿美替尼 | EGFR | CYP3A4 | 说明书提示阿美替尼与利福平联用会导致暴露量显著降低(AUC降低约90%)。药品说明书建议谨慎合用 | A | ||
| 伏美替尼 | EGFR | CYP3A4 | 伏美替尼与强诱导剂或抑制剂联合使用时需谨慎。药品说明书建议谨慎合用 | B | ||
| 贝福替尼 | EGFR | CYP3A4 | 与强诱导剂合并使用可能会导致本品血药浓度降低。药品说明书建议谨慎合用 | B | ||
| 阿法替尼 | EGFR、HER2 | P-gp | 利福平为P-gp诱导剂。上市后研究表明:利福平连续7d,可将阿法替尼的血浆暴露量降低33.8%(AUC)和21.6%(药峰浓度)[ | B | ||
| 达可替尼 | EGFR | CYP2D6、CYP3A4 | 说明书提示利福平作为强CYP3A4诱导剂,合用会导致AUC降低80%,导致治疗失败。药品说明书禁止联用 | A | ||
| 吉非替尼 | EGFR | CYP3A4 | 与CYP3A4强诱导剂利福平合用,吉非替尼的平均AUC比单服时降低83%[ | A | ||
| 厄洛替尼 | EGFR | CYP3A4、CYP1A2 | 说明书提示与CYP3A4强诱导剂利福平合用,导致厄洛替尼的平均AUC降低69%[ | A | ||
| 埃克替尼 | EGFR | CYP2C19、CYP3A4 | 与利福平合用,导致暴露量显著降低。药品说明书建议注意避免潜在的药物相互作用 | A | ||
| 利厄替尼 | EGFR | CYP3A4 | 与利福平合用,导致暴露量显著降低。药品说明书建议避免合用 | A | ||
| 瑞厄替尼 | EGFR | CYP3A4 | 利福平加速代谢,显著减少药物暴露量[ | A | ||
| 瑞齐替尼 | EGFR | CYP3A4、CYP2B6 CYP2C8、CYP2C9 CYP2C19、CYP2D6 | 利福平诱导代谢酶,导致血药浓度下降。药品说明书建议避免合用 | A | ||
| 阿来替尼 | ALK | CYP3A4 | 利福平合并用药对阿来替尼的总暴露量的影响较小[ | B | ||
| 布格替尼 | ALK | CYP2C8、CYP3A4 | 说明书提示利福平合并使用,可使布格替尼的AUC降低80%[ | A | ||
| 洛拉替尼 | ALK | CYP3A4 | 说明书提示本品禁用于正在服用强效CYP3A诱导剂的患者。利福平使洛拉替尼AUC平均值下降85%[ | A | ||
| 恩沙替尼 | ALK | CYP3A4 | 与强诱导剂联合使用可能会导致本品血药浓度降低。慎用利福平。药品说明书建议谨慎合用 | B | ||
| 伊鲁阿克 | ALK | CYP3A4 | 伊鲁阿克尚未完成与CYP3A4抑制剂和诱导剂联用的药物-药物相互作用研究。与利福平合用可能会导致伊鲁阿克血药浓度的降低 | A | ||
| 塞瑞替尼 | ALK | CYP3A4、P-gp | 与强效诱导剂利福平联用可显著降低本品的血浆浓度。药品说明书建议避免合用 | B | ||
| 克唑替尼 | ALK、ROS1、MET | CYP3A4 | 说明书提示利福平合并服用时,克唑替尼的稳态AUC0-Tau降低84%[ | A | ||
| 依奉阿克 | ALK、ROS1、MET | CYP3A4 | 利福平显著降低依奉阿克药物浓度。药品说明书建议避免合用 | A | ||
| 安奈克替尼 | ALK、ROS1、c-Met | CYP3A4 | 与CYP3A4强诱导剂合用会导致血药浓度降低。药品说明书建议避免合用 | A | ||
| 达拉非尼+ 曲美替尼 | BRAFV600 MEK1/2 | CYP2C8、CYP3A4 | 说明书提示本身是CYP3A4诱导剂,利福平增强CYP3A4活性,显著降低达拉非尼浓度,AUC下降34%;药品说明书建议避免合用。曲美替尼与利福平无明显相互作用;药品说明书建议可以合用 | 达拉菲尼B; 曲美替尼C | ||
| 恩曲替尼 | NTRK、ROS1 | CYP3A4 | 说明书提示与强诱导剂利福平合用,可使恩曲替尼的全身暴露量下降77%[ | A | ||
| 拉罗替尼 | NTRK | CYP3A4 | 说明书提示利福平诱导CYP3A4会显著降低其血药浓度(AUC降低81%)。药品说明书建议避免合用 | A | ||
| 瑞普替尼 | ROS1、NTRK | CYP3A4/5 | 强效诱导剂利福平显著降低其血药浓度。药品说明书建议避免合用 | A | ||
| 他雷替尼 | ROS1、NTRK | CYP3A4、CYP2D6、 CYP2C8 | 说明书提示与强诱导剂利福平合用,AUCinf降低86%。药品说明书建议避免合用 | A | ||
| 谷美替尼 | MET | CYP3A4 | CYP3A4为谷美替尼的主要代谢酶,但其对其代谢贡献有限(<5%),提示CYP抑制剂或诱导剂不太可能与临床剂量的谷美替尼发生相互作用。药品说明书建议无需调整剂量 | C | ||
| 伯瑞替尼 | MET | CYP3A4、CYP2C9 | 伯瑞替尼可通过多种代谢酶代谢,但主要通过CYP3A4代谢。说明书提示与强诱导剂利福平合用,可使伯瑞替尼的AUC下降65%。药品说明书建议避免合用 | A | ||
| 特泊替尼 | MET | CYP3A4、P-gp | 强效P-gp诱导剂可能会降低特泊替尼的暴露量。强效CYP诱导剂也可能会降低特泊替尼的暴露量。药品说明书建议避免合用 | A | ||
| 赛沃替尼 | MET | CYP1A2、CYP3A4、 CYP3A5 | 说明书提示与利福平合用会使赛沃替尼AUC降低61%[ | A | ||
| 卡马替尼 | MET | CYP3A4 | 利福平诱导CYP3A4会显著降低其血药浓度。药品说明书建议避免合用或增加剂量 | A | ||
| 氟泽雷塞 | KRAS G12C | 谷胱甘肽S-转移酶 | 氟泽雷赛的主要代谢途径为谷胱甘肽S-转移酶介导的半胱氨酸结合,其对CYP3A4既有潜在抑制作用,也有潜在诱导作用。药品说明书建议可以合用 | C | ||
| 格索雷塞 | KRAS G12C | CYP3A4、CYP2C8 | 利福平显著降低药物暴露量。药品说明书建议避免合用 | A | ||
| 塞普替尼 | RET | CYP3A4 | 说明书提示利福平强效诱导CYP3A4会降低其血药浓度,AUC下降87%。药品说明书建议避免合用 | A | ||
| 普拉替尼 | RET | CYP3A4、CYP2D6、 CYP1A2 | 说明书提示强效CYP3A抑制剂避免与强效CYP3A抑制剂联合用药,AUC下降68%。药品说明书建议避免合用 | A | ||
| 埃万妥单抗 | EGFR、MET | 网状内皮系统降解 | 尚未开展药物相互作用研究 | B | ||
| 贝伐珠单抗 | VEGF-A | 蛋白水解分解代谢 | 与利福平无药代动力学相互作用。药品说明书建议可以合用 | C | ||
| 重组人血管 内皮抑素 | VEGFR2、MMP、 lntegrin等 | 通过蛋白水解代谢 | 与利福平合用未见报道。说明书中无具体提及。根据生物药品特征,其对CYP3A4酶的影响不大,故与利福平合用风险小 | C | ||
| 依沃西单抗 | VEGF-A、PD-1 | 网状内皮系统降解 | 与利福平合用未见相互作用报道。药品说明书建议可以合用 | C | ||
| 安罗替尼 | VEGFR1-3、FGFR、 PDGFR等 | CYP1A2、 CYP3A4/5 | CYP3A4/5诱导剂(利福平、利福布汀、利福喷丁等)可能加速安罗替尼的代谢,降低安罗替尼的血浆浓度。药品说明书建议避免合用 | A | ||
| [1] |
Leung CY, Huang HL, Rahman MM, et al. Cancer incidence attributable to tuberculosis in 2015: global, regional, and national estimates. BMC Cancer, 2020, 20(1): 412. doi:10.1186/s12885-020-06891-5.
pmid: 32398031 |
| [2] | Yu YH, Liao CC, Hsu WH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol, 2011, 6(1): 32-37. doi:10.1097/JTO.0b013e3181fb4fcc. |
| [3] | Liao KM, Shu CC, Liang FW, et al. Risk Factors for Pulmonary Tuberculosis in Patients with Lung Cancer: A Retrospective Cohort Study. J Cancer, 2023, 14(4): 657-664. doi:10.7150/jca.81616. |
| [4] | Qin Y, Chen Y, Chen J, et al. The relationship between previous pulmonary tuberculosis and risk of lung cancer in the future. Infect Agent Cancer, 2022, 17(1): 20. doi:10.1186/s13027-022-00434-2. |
| [5] |
Aoki K. Excess incidence of lung cancer among pulmonary tuberculosis patients. Jpn J Clin Oncol, 1993, 23(4): 205-220.
pmid: 8411734 |
| [6] | Dobler CC, Cheung K, Nguyen J, et al. Risk of tuberculosis in patients with solid cancers and haematological malignancies: a systematic review and meta-analysis. Eur Respir J, 2017, 50(2): 1700157. doi:10.1183/13993003.00157-2017. |
| [7] | McNally E, Ross C, Gleeson LE. The tuberculous pleural effusion. Breathe (Sheff), 2023, 19(4): 230143. doi:10.1183/20734735.0143-2023. |
| [8] |
Zhang F, Qi F, Han Y, et al. Clinical and imaging features of co-existent pulmonary tuberculosis and lung cancer: a population-based matching study in China. BMC Cancer, 2025, 25(1): 89. doi:10.1186/s12885-024-13350-y.
pmid: 39815214 |
| [9] |
Ma J, Xia D, Hu J, et al. Predictive Role of Serum Tumor Markers in Diagnosis of Pulmonary Tuberculosis. Iran J Public Health, 2016, 45(4): 435-440.
pmid: 27252912 |
| [10] |
Zhao P, Yu Q, Zhang A, et al. Serum CA-125 for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. BMC Infect Dis, 2021, 21(1): 1091. doi:10.1186/s12879-021-06772-7.
pmid: 34688261 |
| [11] |
Jia H, Zhang L, Wang B. The Value of Combination Analysis of Tumor Biomarkers for Early Differentiating Diagnosis of Lung Cancer and Pulmonary Tuberculosis. Ann Clin Lab Sci, 2019, 49(5): 645-649.
pmid: 31611208 |
| [12] | Kwon JJ, Factora TD, Dey S, et al. A Systematic Review of miR-29 in Cancer. Mol Ther Oncolytics, 2019, 12: 173-194. doi:10.1016/j.omto.2018.12.011. |
| [13] | 中华医学会检验医学分会, 国家卫生健康委员会临床检验中心. 液体活检在临床肿瘤诊疗应用和医学检验实践中的专家共识. 中华检验医学杂志, 2018, 41(10): 724-733. doi:10.3760/cma.j.issn.1009-9158.2018.10.006. |
| [14] |
Kim YI, Goo JM, Kim HY, et al. Coexisting bronchogenic carcinoma and pulmonary tuberculosis in the same lobe: radiologic findings and clinical significance. Korean J Radiol, 2001, 2(3): 138-144. doi:10.3348/kjr.2001.2.3.138.
pmid: 11752984 |
| [15] |
Hwang IK, Paik SS, Lee SH. Impact of Pulmonary Tuberculosis on the EGFR Mutational Status and Clinical Outcome in Patients with Lung Adenocarcinoma. Cancer Res Treat, 2019, 51(1): 158-168. doi:10.4143/crt.2018.084.
pmid: 29621876 |
| [16] |
Im JG, Itoh H, Han MC. CT of pulmonary tuberculosis. Semin Ultrasound CT MR, 1995, 16(5): 420-434. doi:10.1016/0887-2171(95)90029-2.
pmid: 8527173 |
| [17] | Wood DE, Kazerooni EA, Aberle DR, et al. NCCN Guidelines Insights: Lung Cancer Screening, Version 1.2025. J Natl Compr Canc Netw, 2025, 23(1): e250002. doi:10.6004/jnccn.2025.0002. |
| [18] |
Christensen J, Prosper AE, Wu CC, et al. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. Chest, 2024, 165(3):738-753. doi:10.1016/j.chest.2023.10.028.
pmid: 38300206 |
| [19] | Cabrera-Sanchez J, Cuba V, Vega V, et al. Lung cancer occurrence after an episode of tuberculosis: a systematic review and meta-analysis. Eur Respir Rev, 2022, 31(165): 220025. doi:10.1183/16000617.0025-2022. |
| [20] | 中华医学会肿瘤学分会. 中华医学会肺癌临床诊疗指南(2024版). 中华肿瘤杂志, 2024, 46(9):805-843. doi:10.3760/cma.j.cn112137-20240511-01092. |
| [21] | Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax, 2011, 66 Suppl 3:iii1-21. doi:10.1136/thoraxjnl-2011-200713. |
| [22] | 中华医学会呼吸病学分会介入呼吸病学学组. 成人诊断性可弯曲支气管镜检查术应用指南(2019年版). 中华结核和呼吸杂志, 2019, 42(8):573-590. doi:10.3760/cma.j.issn.1001-0939.2019.08.005. |
| [23] | 中华医学会呼吸病分会. 诊断性可弯曲支气管镜应用指南(2008年版). 中华结核和呼吸杂志, 2008, 31(1):14-17. doi:10.3321/j.issn:1001-0939.2008.01.007. |
| [24] | 彭爱梅, 李明, 张国良, 等. 荧光支气管镜评估中央型肺癌浸润范围及指导治疗的价值. 中华内科杂志, 2015, 54(1): 40-43. doi:10.3760/cma.j.issn.0578-1426.2015.01.011. |
| [25] | 陈众博, 虞亦鸣, 孙士芳, 等. 窄带成像联合自荧光支气管镜对中央型肺癌的诊断价值. 中华结核和呼吸杂志, 2014, 37(3):184-187. doi:10.3760/cma.j.issn.1001-0939.2014.03.008. |
| [26] | 罗为展, 钟长镐, 陈愉, 等. 肺癌体内共聚焦激光显微内镜成像的初步观察. 中华结核和呼吸杂志, 2015, 38(10):792-793. doi:10.3760/cma.j.issn.1001-0939.2015.10.022. |
| [27] | Su ZQ, Guan WJ, Li SY, et al. Evaluation of the Normal Airway Morphology Using Optical Coherence Tomography. Chest, 2019, 156(5): 915-925. doi:10.1016/j.chest.2019.06.009. |
| [28] | 王艳, 许小毛, 周为, 等. 超声引导下经支气管镜针吸活检对老年肺门和/或纵隔占位性病变患者的诊断价值及安全性研究. 中华老年医学杂志, 2024, 43(4):438-443. doi:10.3760/cma.j.issn.0254-9026.2024.04.005. |
| [29] | 孙加源. 重视肺外周病变经支气管镜诊断技术. 中华结核和呼吸杂志, 2021, 44(12):1040-1042. doi:10.3760/cma.j.cn112147-20210830-00608. |
| [30] | 田森, 王新宇, 黄海东, 等. 支气管镜技术在肺外周病变诊断中的研究进展. 中华内科杂志, 2023, 62(11): 1346-1352. doi:10.3760/cma.j.cn112138-20221125-00886. |
| [31] | 李梦远, 陈青霞, 卢以杰, 等. 超细支气管镜和常规支气管镜对周围型肺病变的诊断价值研究. 中华结核和呼吸杂志, 2024, 43(4):332-338. doi:10.3760/cma.j.cn112147-20231015-00234. |
| [32] | 中国抗癌协会肿瘤介入学专业委员会, 中国抗癌协会肿瘤介入学专业委员会胸部肿瘤诊疗专家委员会. 胸部肿瘤经皮穿刺活检中国专家共识(2020版). 中华医学杂志, 2021, 101(3):185-198. doi:10.3760/cma.j.cn112137-20200907-02576. |
| [33] | Huang MD, Weng HH, Hsu SL, et al. Accuracy and complications of CT-guided pulmonary core biopsy in small nodules: a single-center experience. Cancer Imaging, 2019, 19(1): 51. doi:10.1186/s40644-019-0240-6. |
| [34] | Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol, 2011, 196(6): W678-82. doi:10.2214/AJR.10.4659. |
| [35] | Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax, 2010, 65 Suppl 2: ii54-60. doi:10.1136/thx.2010.137018. |
| [36] |
Anevlavis S, Froudarakis ME. Advances in pleuroscopy. Clin Respir J, 2018, 12(3): 839-847. doi:10.1111/crj.12597.
pmid: 27997741 |
| [37] | College of American Pathologists. Ptotocl for the examination of resection specimens from patients with primary non-small cell carcinoma, small cel carcinoma, or carcinoid tumor of the lung (version 5.0.0.0)[R/OL].[2025-06-30]. https://documents.cap.org/protocols/Lung_5.0.0.0.REL.CAPCP.pdf. |
| [38] | The WHO Classification of Tumors Editorial Board. WHO classification of tumors of thoracic tumours. 5th ed. Geneva: World Health Organization, 2020. |
| [39] | 中国医师协会呼吸医师分会呼吸病理工作委员会及共识编写专家组. 肺肉芽肿性疾病病理诊断原则及流程专家建议. 中华病理学杂志, 2021, 50(7): 719-727. doi:10.3760/cma.j.cn112151-20210128-00092. |
| [40] |
Dagaonkar RS, Choong CV, Asmat AB, et al. Significance of coexistent granulomatous inflammation and lung cancer. J Clin Pathol, 2017, 70(4): 337-341. doi:10.1136/jclinpath-2016-203868.
pmid: 27646525 |
| [41] | Yakar F, Yakar A, Büyükpınarbaşılı N, et al. Does Every Necrotizing Granulomatous Inflammation Identified by NSCLC Resection Material Require Treatment?. Med Sci Monit, 2016, 22: 1218-1222. doi:10.12659/msm.897638. |
| [42] |
Steinfort DP, Irving LB. Sarcoidal reactions in regional lymph nodes of patients with non-small cell lung cancer: incidence and implications for minimally invasive staging with endobronchial ultrasound. Lung Cancer, 2009, 66(3): 305-308. doi:10.1016/j.lungcan.2009.03.001.
pmid: 19329219 |
| [43] |
Tomimaru Y, Higashiyama M, Okami J, et al. Surgical results of lung cancer with sarcoid reaction in regional lymph nodes. Jpn J Clin Oncol, 2007, 37(2): 90-95. doi:10.1093/jjco/hyl141.
pmid: 17272320 |
| [44] |
Steinfort DP, Tsui A, Grieve J, et al. Sarcoidal reactions in regional lymph nodes of patients with early stage non-small cell lung cancer predict improved disease-free survival: a pilot case-control study. Hum Pathol, 2012, 43(3): 333-338. doi:10.1016/j.humpath.2011.05.006.
pmid: 21835432 |
| [45] |
Kamiyoshihara M, Hirai T, Kawashima O, et al. Sarcoid reactions in primary pulmonary carcinoma: report of seven cases. Oncol Rep, 1998, 5(1): 177-180. doi:10.3892/or.5.1.177.
pmid: 9458317 |
| [46] |
Hirashima T, Tamura Y, Han Y, et al. Efficacy and safety of concurrent anti-Cancer and anti-tuberculosis chemotherapy in Cancer patients with active Mycobacterium tuberculosis: a retrospective study. BMC Cancer, 2018, 18(1): 975. doi:10.1186/s12885-018-4889-1.
pmid: 30314434 |
| [47] |
Ryan AM, Power DG, Daly L, et al. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc, 2016, 75(2): 199-211. doi:10.1017/S002966511500419X.
pmid: 26786393 |
| [48] | Ho JC, Leung CC. Management of co-existent tuberculosis and lung cancer. Lung Cancer, 2018, 122: 83-87. doi:10.1016/j.lungcan.2018.05.030. |
| [49] | US Food and Drug Administration. Drug development and drug interactions: table of substrates, inhibitors and inducers[EB/OL]. [2025-06-29]. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers. |
| [50] |
Elmeliegy M, Vourvahis M, Guo C, et al. Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: Review of Clinical Drug-Drug Interaction Studies. Clin Pharmacokinet, 2020, 59(6): 699-714. doi:10.1007/s40262-020-00867-1.
pmid: 32052379 |
| [51] |
Chambers HF. Rifabutin to the Rescue?. J Infect Dis, 2020, 222(9): 1422-1424. doi:10.1093/infdis/jiaa403.
pmid: 32914842 |
| [52] | Tuloup V, France M, Garreau R, et al. Model-Based Compara-tive Analysis of Rifampicin and Rifabutin Drug-Drug Interaction Profile. Antimicrob Agents Chemother, 2021, 65(9): e0104321. doi:10.1128/AAC.01043-21. |
| [53] | Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med, 2018, 378(2): 113-125. doi:10.1056/NEJMoa1713137. |
| [54] | Lu S, Dong X, Jian H, et al. AENEAS: A Randomized Phase Ⅲ Trial of Aumolertinib Versus Gefitinib as First-Line Therapy for Locally Advanced or MetastaticNon-Small-Cell Lung Cancer With EGFR Exon 19 Deletion or L858R Mutations. J Clin Oncol, 2022, 40(27): 3162-3171. doi:10.1200/JCO.21.02641. |
| [55] |
Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med, 2022, 10(11): 1019-1028. doi:10.1016/S2213-2600(22)00168-0.
pmid: 35662408 |
| [56] | Lu S, Zhou J, Jian H, et al. Befotertinib (D-0316) versus icotinib as first-line therapy for patients with EGFR-mutated locally advanced or metastatic non-small-cell lung cancer: a multicentre, open-label, randomised phase 3 study. Lancet Respir Med, 2023, 11(10): 905-915. doi:10.1016/S2213-2600(23)00183-2. |
| [57] |
Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol, 2016, 17(5): 577-589. doi:10.1016/S1470-2045(16)30033-X.
pmid: 27083334 |
| [58] | 仲佳, 王洁. 表皮生长因子受体酪氨酸激酶抑制剂药物相互作用概述. 中华肿瘤杂志, 2022, 44(7): 717-724. doi:10.3760/cma.j.cn112152-20210909-00687. |
| [59] |
Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol, 2015, 16(7): 830-838. doi:10.1016/S1470-2045(15)00026-1.
pmid: 26051236 |
| [60] | Morcos PN, Cleary Y, Guerini E, et al. Clinical Drug-Drug Interactions Through Cytochrome P 450 3A (CYP3A) for the Selective ALK Inhibitor Alectinib. Clin Pharmacol Drug Dev, 2017, 6(3): 280-291. doi:10.1002/cpdd.298. |
| [61] |
Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol, 2016, 17(7): 984-993. doi:10.1016/S1470-2045(16)30146-2.
pmid: 27283860 |
| [62] | US Food and Drug Administration. TAGRISSOTM(osimertinib) tablet, for oral use[EB/OL]. [2025-06-29]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208065s000lbl.pdf. |
| [63] |
Swaisland HC, Ranson M, Smith RP, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet, 2005, 44(10): 1067-1081. doi:10.2165/00003088-200544100-00005.
pmid: 16176119 |
| [64] | Hamilton M, Wolf JL, Drolet DW, et al. The effect of rifampicin, a prototypical CYP3A 4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer Chemother Pharmacol, 2014, 73(3): 613-621. doi:10.1007/s00280-014-2390-3. |
| [65] | Wind S, Giessmann T, Jungnik A, et al. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig, 2014, 34(3): 173-182. doi:10.1007/s40261-013-0161-2. |
| [66] | Tugnait M, Gupta N, Hanley MJ, et al. Effects of Strong CYP2C 8 or CYP3A Inhibition and CYP3A Induction on the Pharmacokinetics of Brigatinib, an Oral Anaplastic Lymphoma Kinase Inhibitor, in Healthy Volunteers. Clin Pharmacol Drug Dev, 2020, 9(2): 214-223. doi:10.1002/cpdd.723. |
| [67] |
Chen J, Xu H, Pawlak S, et al. The Effect of Rifampin on the Pharmacokinetics and Safety of Lorlatinib: Results of a Phase One, Open-Label, Crossover Study in Healthy Participants. Adv Ther, 2020, 37(2): 745-758. doi:10.1007/s12325-019-01198-9.
pmid: 31863284 |
| [68] | Xu H, O’Gorman M, Tan W, et al. The effects of ketocona-zole and rifampin on the single-dose pharmacokinetics of crizotinib in healthy subjects. Eur J Clin Pharmacol, 2015, 71(12): 1441-1449. doi:10.1007/s00228-015-1945-5. |
| [69] | Meneses-Lorente G, Fowler S, Guerini E, et al. In vitro and clinical investigations to determine the drug-drug interaction potential of entrectinib, a small molecule inhibitor of neurotrophic tyrosine receptor kinase (NTRK). Invest New Drugs, 2022, 40(1): 68-80. doi:10.1007/s10637-021-01156-9. |
| [70] | Ren S, Vishwanathan K, Cantarini M, et al. Clinical evaluation of the potential drug-drug interactions of savolitinib: Interaction with rifampicin, itraconazole, famotidine or midazolam. Br J Clin Pharmacol, 2022, 88(2): 655-668. doi:10.1111/bcp.14994. |
| [71] |
Chai M, Shi Q. The effect of anti-cancer and anti-tuberculosis treatments in lung cancer patients with active tuberculosis: a retrospective analysis. BMC Cancer, 2020, 20(1): 1121. doi:10.1186/s12885-020-07622-6.
pmid: 33213414 |
| [72] |
Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J, 2022, 43(41): 4229-4361. doi:10.1093/eurheartj/ehac244.
pmid: 36017568 |
| [73] | 中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)肿瘤心脏病学指南. 北京: 人民卫生出版社, 2023. |
| [74] | 中国防痨协会, 《中国防痨杂志》编辑委员会, 首都医科大学附属北京胸科医院. 抗结核药物所致QTc间期延长临床监测和管理专家共识. 中国防痨杂志, 2024, 46(1):8-17. doi:10.19982/j.issn.1000-6621.20230271. |
| [75] | Liu K, Wang D, Yao C, et al. Increased Tuberculosis Incidence Due to Immunotherapy Based on PD-1 and PD-L 1 Blockade: A Systematic Review and Meta-Analysis. Front Immunol, 2022, 13: 727220. doi:10.3389/fimmu.2022.727220. |
| [76] | Kim HW, Kim JS, Lee SH. Incidence of tuberculosis in advanced lung cancer patients treated with immune checkpoint inhibitors-A nationwide population-based cohort study. Lung Cancer, 2021, 158: 107-114. doi:10.1016/j.lungcan.2021.05.034. |
| [77] | Bae S, Kim YJ, Kim MJ, et al. Risk of tuberculosis in patients with cancer treated with immune checkpoint inhibitors: a nationwide observational study. J Immunother Cancer, 2021, 9(9): e002960. doi:10.1136/jitc-2021-002960. |
| [78] | 北京医师协会呼吸内科专科医师分会咯血诊治专家共识编写组. 咯血诊治专家共识. 中国呼吸与危重监护杂志, 2020, 19(1):1-11. doi:10.7507/1671-6205.201911006. |
| [79] | 中华医学会, 中华医学会杂志社, 中华医学会全科医学分会, 等. 肺结核基层诊疗指南(2018年). 中华全科医师杂志, 2019, 18(8):709-717. doi:10.3760/cma.j.issn.1671-7368.2019.08.002. |
| [80] | 贝伐珠单抗注射液中文说明书. 2024年版. 信达生物制药(苏州)有限公司. |
| [81] | 盐酸安罗替尼胶囊中文说明书. 2023年版. 正大天晴药业集团股份有限公司. |
| [82] | 依沃西单抗注射液中文说明书. 2025年版. 康方赛诺医药有限公司. |
| [83] |
HARMONi-A Study Investigators, Fang W, Zhao Y, et al. Ivonescimab Plus Chemotherapy in Non-Small Cell Lung Cancer With EGFR Variant: A Randomized Clinical Trial. JAMA, 2024, 332(7): 561-570. doi:10.1001/jama.2024.10613.
pmid: 38820549 |
| [84] |
Xiong A, Wang L, Chen J, et al. Ivonescimab versus pembrolizumab for PD-L1-positive non-small cell lung cancer (HARMONi-2): a randomised, double-blind, phase 3 study in China. Lancet, 2025, 405(10481): 839-849. doi:10.1016/S0140-6736(24)02722-3.
pmid: 40057343 |
| [85] | Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol, 2022, 23(9): 1167-1179. doi:10.1016/S1470-2045(22)00382-5. |
| [86] | 中国临床肿瘤学会指南工作委员会. CSCO非小细胞肺癌诊疗指南2025. 北京: 人民卫生出版社, 2025. |
| [87] | National Comprehensive Cancer Network. Small Cell Lung Cancer (Version 4.2025). 2025-01-13. |
| [88] | National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 7.2025). 2025-07-10. |
| [89] | 杨海霞, 张小燕, 黄毅, 等. 活动性肺结核合并胸部肿瘤调强放射治疗的安全性及疗效分析. 中国防痨杂志, 2025, 47(3):312-321. doi:10.19982/j.issn.1000-6621.20240450. |
| [90] | 梁香存, 王庆, 梁凯, 等. Ⅲ期非小细胞肺癌合并继发型肺结核放疗时机的研究. 河北医药, 2015, 37(20):3048-3051. doi:10.3969/j.issn.1002-7386.2015.20.002. |
| [91] | 罗林紫, 肖阳宝. 硬质支气管镜联合可弯曲支气管镜治疗淋巴结瘘型气管支气管结核所致中心气道狭窄2例. 中华结核和呼吸杂志, 2024, 47(2): 137-140.doi:10.3760/cma.j.cn112147-20230905-00139. |
| [92] | 北京健康促进会呼吸及肿瘤介入诊疗联盟. 恶性中心气道狭窄经支气管镜介入诊疗专家共识. 中华肺部疾病杂志(电子版), 2017, 10(6): 647-654. doi:10.3877/cma.j.issn.1674-6902.2017.06.004. |
| [93] | Tang A, Ahmad U, Raja S, et al. How Much Delay Matters? How Time to Treatment Impacts Overall Survival in Early Stage Lung Cancer. Ann Surg, 2023, 277(4): e941-e947. doi:10.1097/SLA.0000000000005307. |
| [94] | Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline-Pretomanid-Linezolid Regimens for Drug-Resistant Tuberculosis. N Engl J Med, 2022, 387(9): 810-823. doi:10.1056/NEJMoa2119430. |
| [95] |
Evman S, Baysungur V, Alpay L, et al. Management and Surgical Outcomes of Concurrent Tuberculosis and Lung Cancer. Thorac Cardiovasc Surg, 2017, 65(7): 542-545. doi:10.1055/s-0036-1583167.
pmid: 27111500 |
| [96] | World Health Organization. WHO consolidated guidelines on tuberculosis: Module 1: Prevention-Tuberculosis preventive treatment, second edition. Geneva: World Health Organization, 2024. |
| [97] | 中华医学会结核病学分会. 结核分枝杆菌γ-干扰素释放试验及临床应用专家意见(2021年版). 中华结核和呼吸杂志, 2022, 45(2): 143-150. doi:10.3760/cma.j.cn112147-20211110-00794. |
| [98] |
Fujita K, Elkington P, Redelman-Sidi G, et al. Serial interferon-gamma release assay in lung cancer patients receiving immune checkpoint inhibitors: a prospective cohort study. Cancer Immunol Immunother, 2022, 71(11): 2757-2764. doi:10.1007/s00262-022-03198-1.
pmid: 35429244 |
| [99] | 徐彩红, 赵雁林. 中国结核病预防性治疗指南. 北京: 人民卫生出版社, 2023. |
| [100] | Parker CS, Siracuse CG, Litle VR. Identifying lung cancer in patients with active pulmonary tuberculosis. J Thorac Dis, 2018, 10(Suppl 28): S3392-S3397. doi:10.21037/jtd.2018.07.11. |
| [1] | 杜润泽, 艾尔帕提·玉素甫, 董士铭, 徐韬, 蔡晓宇, 王婷, 牙克甫·阿卜力孜, 盛伟斌, 买尔旦·买买提. Lasso-logistic回归在感染性脊柱炎鉴别诊断中的应用[J]. 中国防痨杂志, 2025, 47(S1): 1-4. |
| [2] | 杜润泽, 董士铭, 艾尔帕提·玉素甫, 徐韬, 蔡晓宇, 王婷, 牙克甫·阿卜力孜, 盛伟斌, 买尔旦·买买提. 布鲁氏菌性脊柱炎的精确诊断:多因素Logistic回归预测模型的构建与验证[J]. 中国防痨杂志, 2025, 47(S1): 17-20. |
| [3] | 李春晶, 李莉, 梁雪薇, 袁嘉瑞, 林静. CT三维重建在骨结核术后效果评估中的价值研究[J]. 中国防痨杂志, 2025, 47(S1): 47-49. |
| [4] | 周巍, 任鑫. 探讨多层螺旋CT在肺结核诊断和鉴别诊断中的临床应用价值[J]. 中国防痨杂志, 2025, 47(S1): 71-73. |
| [5] | 暴建萍. 二维高频超声对颈部淋巴结结核的诊断价值评价[J]. 中国防痨杂志, 2025, 47(S1): 141-143. |
| [6] | 胡松. 多层螺旋CT在早期肺癌诊断中的应用价值[J]. 中国防痨杂志, 2025, 47(S1): 157-159. |
| [7] | 王珏, 姚瀚鑫, 孙雪娟, 冯丹, 吴秒一, 张铭锦, 马昕启. 结核病分子诊断技术的进展[J]. 中国防痨杂志, 2025, 47(S1): 366-371. |
| [8] | 杨洁, 贾如. 胸部皮肤病变与肺结核的关联性研究进展[J]. 中国防痨杂志, 2025, 47(S1): 393-395. |
| [9] | 国家感染性疾病临床医学研究中心, 北京大学深圳医院, 深圳市炎症与免疫性疾病重点实验室, 深圳市第三人民医院, 结节性红斑病因诊断的专家共识小组. 结节性红斑病因诊断的专家共识(2025版)[J]. 中国防痨杂志, 2025, 47(9): 1126-1134. |
| [10] | 赖晓宇, 段鸿飞, 陈珣珣, 郭卉欣, 廖庆华, 陈茜, 梁丹. 结核性葡萄膜炎临床特征、诊断策略与分级标准研究进展[J]. 中国防痨杂志, 2025, 47(9): 1204-1211. |
| [11] | 樊瑞芳, 代小伟, 杨新宇, 陈双双, 陈昊, 于兰, 赵琰枫, 李传友, 王嫩寒. 荧光PCR探针熔解曲线技术和DNA微阵列芯片技术鉴定分枝杆菌菌种的研究[J]. 中国防痨杂志, 2025, 47(8): 1031-1037. |
| [12] | 焦家欢, 孙长峰, 吴刚, 黄富礼, 盛云建. 基于机器学习算法的诊断模型对结核性胸腔积液的应用价值[J]. 中国防痨杂志, 2025, 47(8): 1053-1061. |
| [13] | 朱庆东, 赵春艳, 谢周华, 宋树林, 宋畅. 基于人工智能的CT影像组学在结核病诊断和治疗反应监测中应用的研究进展[J]. 中国防痨杂志, 2025, 47(8): 1068-1076. |
| [14] | 朱清玉, 刘家云, 龙铟. 细胞外囊泡与结核病诊断的研究进展[J]. 中国防痨杂志, 2025, 47(8): 1077-1084. |
| [15] | 温建芸, 刘晓婷, 冯晓勤, 吴学东, 陈丽白. 维奈克拉联合个体化抗结核方案成功治疗儿童初发急性髓系白血病合并活动性结核病一例[J]. 中国防痨杂志, 2025, 47(8): 1085-1088. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备11010202007215号
ip访问总数: ip当日访问总数: 当前在线人数:
京公网安备11010202007215号
ip访问总数: ip当日访问总数: 当前在线人数:
 本作品遵循Creative Commons Attribution 3.0 License授权许可
本作品遵循Creative Commons Attribution 3.0 License授权许可